Introduction to Liver MRI for Surgeons
- Diagnosis of Metastatic Liver Cancer -
Images Required by Surgeons
Treatment strategies for colorectal liver metastasis
Liver metastases from colorectal cancer are often resected and a curative resection often results in long-term survival. Advances in chemotherapy for colorectal cancer have improved the response rate to chemotherapy in patients with advanced colorectal cancer, but colorectal liver metastasis can be difficult to cure completely with chemotherapy alone and requires a multimodal approach including liver resection.
Chemotherapy can convert a previously unresectable liver metastasis to resectable one (conversion), and outcomes among patients who undergo resection of converted metastases compare favorably with patients who undergo the same procedure for initially resectable metastases2). In a prospective multi-center study, the authors (Hatano et al.) treated patients with KRAS wild-type unresectable liver metastasis with an anti-EGFR antibody drug (cetuximab) and KRAS mutant unresectable liver metastasis with an anti-angiogenic agent (bevacizumab)3). The overall response rate was 64.7% and 67.6% of patients with unresectable liver metastasis who underwent liver resection. Patients with conversion and R0/1 resection recorded significantly better five-year survival than patients with conversion and non R0/1 resection (66.3% vs. 8.3%)4).
Investigations to determine treatment strategy and surgical procedure
When investigating the resectability of colorectal liver metastasis, as well as using multidetector CT (MDCT) or PET/CT to determine the resectability of the primary lesion and the presence of non-liver metastases, identifying the presence of liver metastases is of vital importance. When MDCT does not reveal factors that entirely rule out surgical intervention, EOB-MRI is used to check for small lesions not detectable by CT. EOB-MRI is essential in patients with multiple lesions, as adding EOB-MRI to MDCT often results in the detection of previously undetected small lesions.
Based on tumors detected by MDCT and EOB-MRI, reconstructed images are prepared using MDCT, and the future liver remnant (FLR) is calculated and used to decide on a surgical procedure.
Intraoperative perfluorobutane-enhanced ultrasonography is indispensable for complex liver resections as it not only finds deep, small lesions, but is also excellent for verifying tumor boundaries.
Precautions for liver resection after conversion
Two problematic aspects of liver resection after conversion are when to perform liver resection and how to address nodules that disappear on images (radiological CR).
Good communication with the liver surgery specialist is beneficial when considering indications for liver resection after conversion, as the best time for resection is when the specialist first deicides that resection is possible. Arbitrary prolongation of chemotherapy is likely to result in chemotherapy-induced liver damage. Identifying liver metastases before chemotherapy is also important to ensure no residual tumor remains after resection and EOB-MRI before treatment is recommended for this purpose. These practices are required because appropriate chemotherapy converts approx. two-thirds of unresectable cases into resectable cases3).
Radiological CR is not true CR5), as 20–30% of nodules remain viable even after radiological CR diagnosed based on both EOB-MRI and contrast-enhanced ultrasonography6).
Patients difficult to diagnose by CT
Images provided by: Hamamatsu University School of Medicine
Male patient in his 70s
History of present illness: The patient was diagnosed with ascending colon cancer and associated unresectable liver metastasis in September 201X and underwent laparoscopic right hemicolectomy. After 11 courses of mFOLFOXIRI + bevacizumab therapy, a new lesion was discovered in segment 4 of the liver. After a further 4 courses of FOLFIRI plus AFL therapy, the patient was referred for surgical intervention.
Fig. 2 Investigation for tumors with EOB-MRI before chemotherapy
Purpose: To identify tumors undetectable by CT before chemotherapy; also useful for evaluating lesion disappearance after chemotherapy.
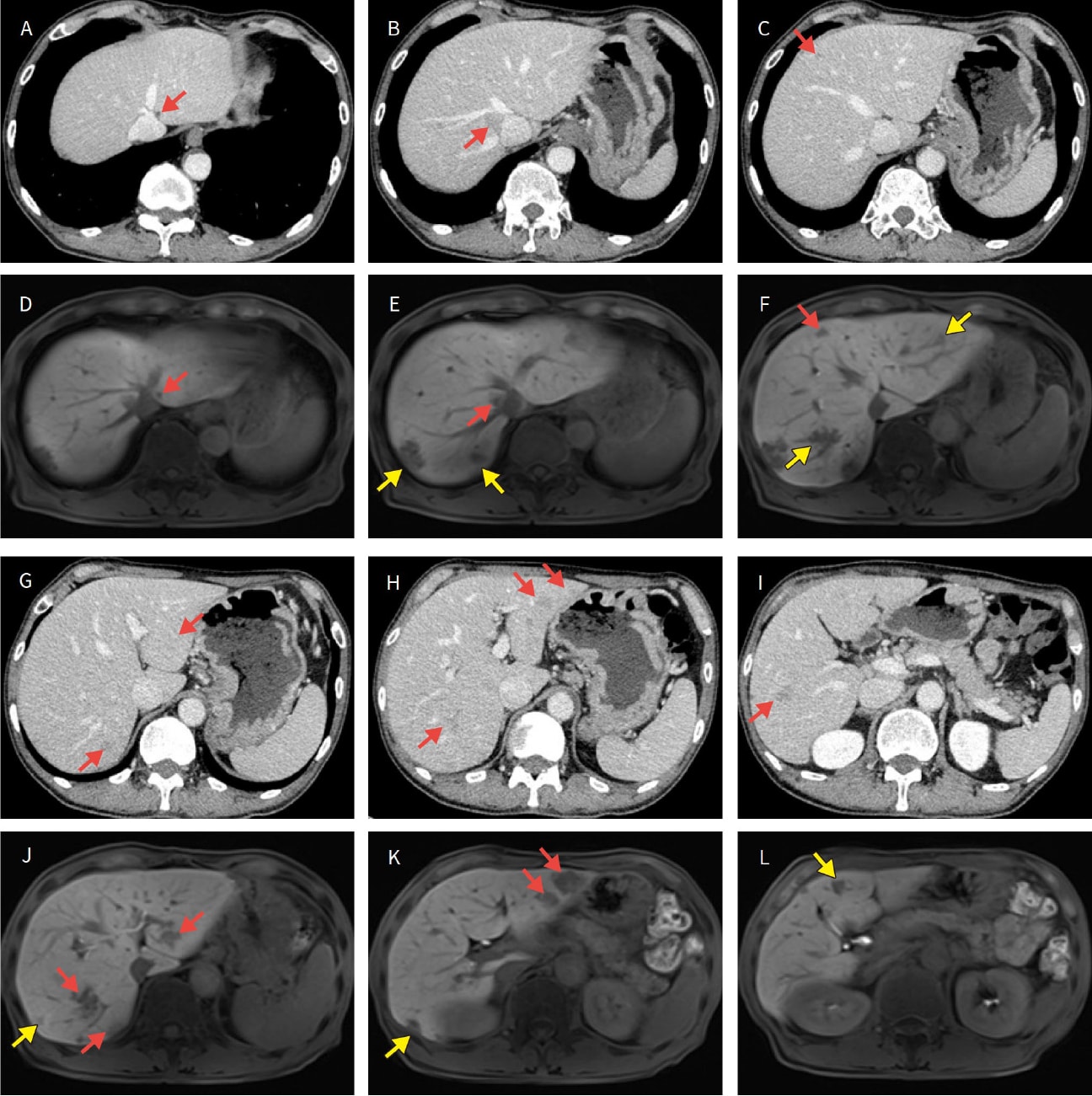
A to C and G to I: Contrast-enhanced CT. D to F and J to L: EOB-MRI. : Tumors visible on both CT and MRI.
: Tumors only visible on EOB-MRI.
Fig. 3 Evaluation by EOB-MRI after chemotherapy
Purpose: To evaluate new lesions and lesions that disappeared after chemotherapy.
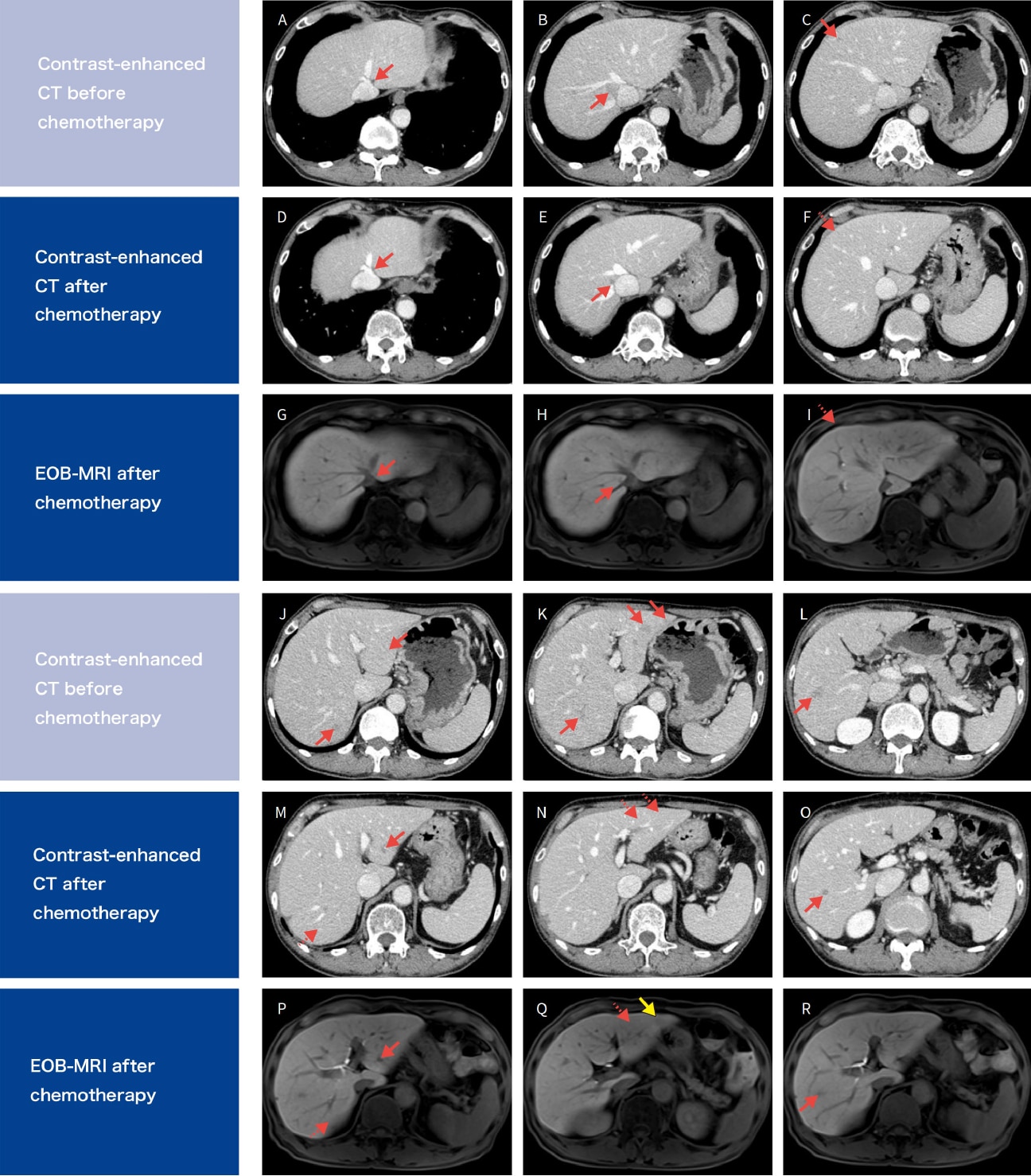
Contrast-enhanced CT before chemotherapy reprinted from Fig. 2. : Visible tumors.
: Disappeared on CT or EOB-MRI.
: Tumors only visible on EOB-MRI
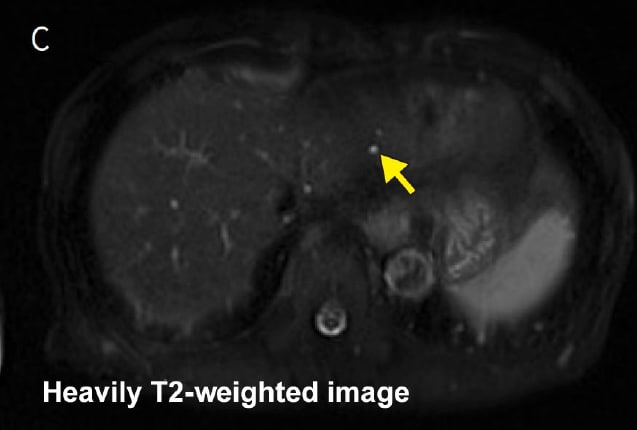
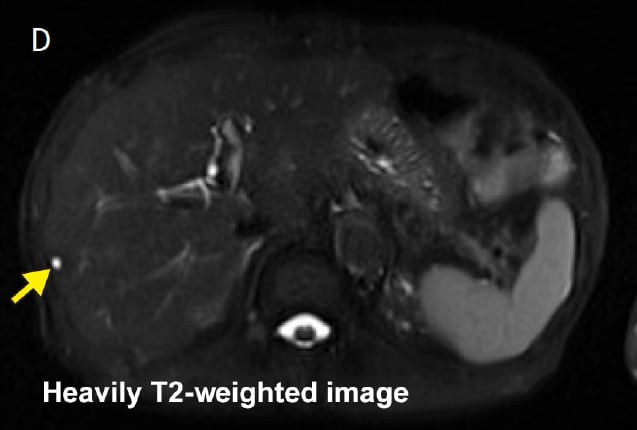
Fig. 4 Differentiating liver cysts by comparing EOB-MRI (hepatobiliary phase) and heavily T2-weighted imaging
Purpose: To select the appropriate surgical procedure (verify targets for resection)
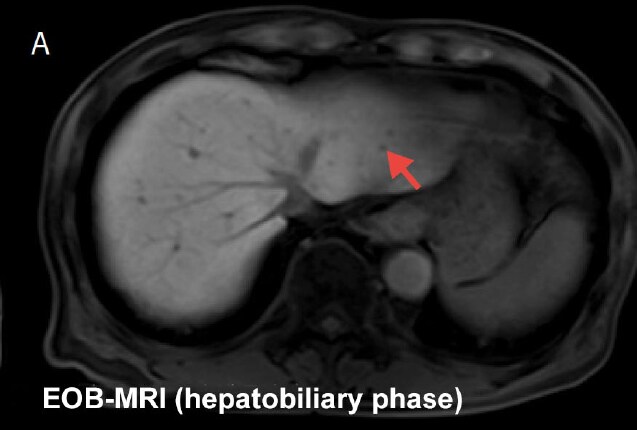
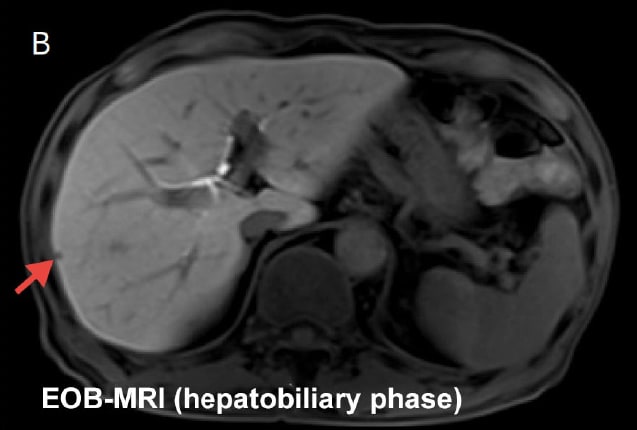
: Hypointense in hepatobiliary phase.
: Hyperintense in heavily T2-weighted image.
Fig. 5 Evaluating the relationship between tumors and vessels
Purpose: To select the appropriate surgical procedure (whether combined vessel resection is required)
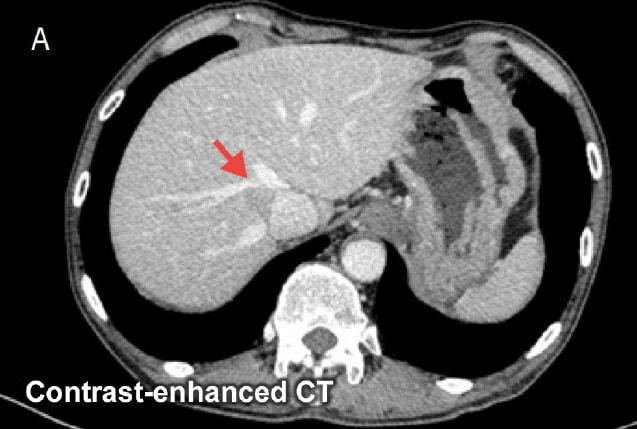
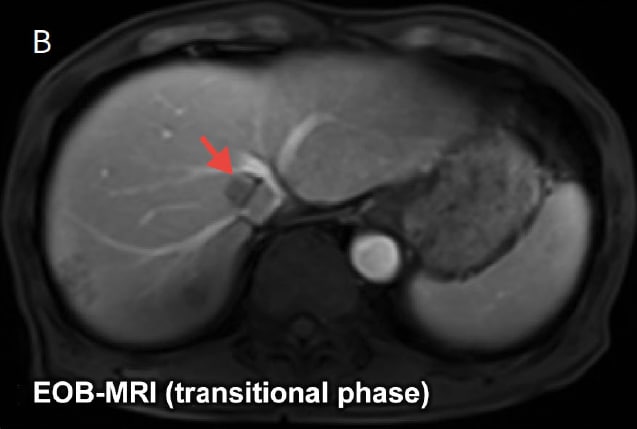
Relationship between tumor and inferior vena cava and hepatic vein is unclear in contrast-enhanced CT image (A). EOB-MRI transitional phase image (B) reveals no infiltration of inferior vena cava or hepatic vein.
Fig. 6 Placement of tumors confirmed by EOB-MRI on 3D-CT images
Purpose: Simulate surgery
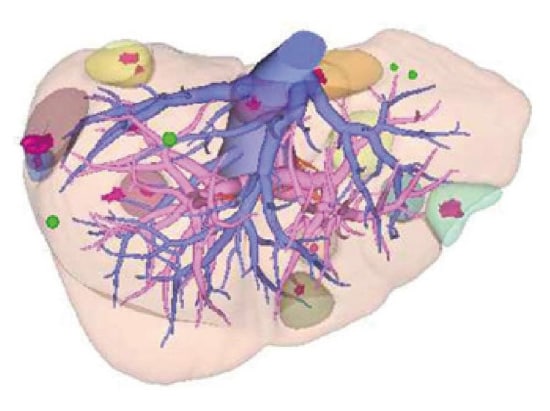
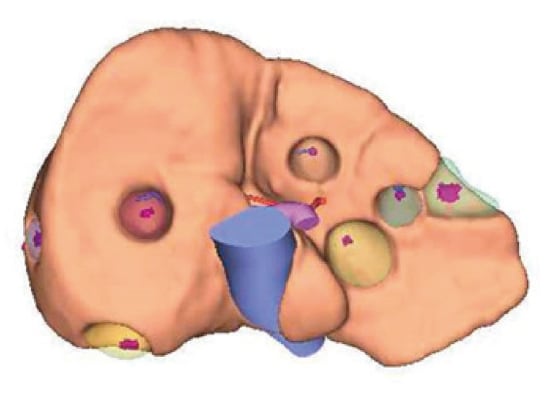
Surgical plan: Planned resection of 10 or 11 locations in liver and radiofrequency ablation of 2 locations in liver.


