Practical report of use for discrimination between hepatocellular carcinoma (HCC) and hemodynamic abnormalities: Discriminatory diagnosis of HCC versus hemodynamic abnormalities
Graduate School of Medicine, Yamaguchi University
Dept. of Radiology
Drs. Masahiro Tanabe, Takeshi Fujita, Naofumi Matsunaga
DATE : 2021
Discriminatory diagnosis of HCC versus hemodynamic abnormalities

Patient’s background and objectives of magnetic resonance imaging (MRI)
Male, 70s.
The patient had chronic hepatitis C, with elevated PIVKA-II (7,900 mAU/mL), and ultrasonography showed a mass approxi-mately 2 cm in diameter in S8 of the liver.
Computed tomography (CT) and MRI were performed to determine the HCC stage. The interval between CT and MRI was 18 days.
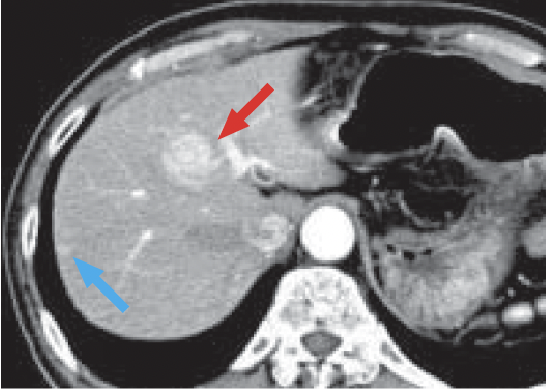
a) Arterial phase
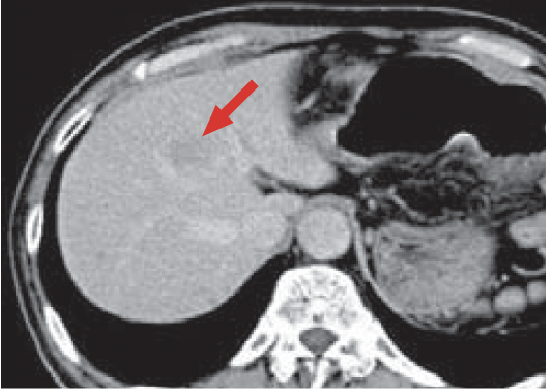
b) Late phase
Multidetector CT
In the arterial phase (a), a dark-stained mass approximately 2 cm in diameter was found in S8 of the liver (red arrow), and a wedge-shaped, dark-stained region was found in the periphery of S7/8 of the liver (blue arrow). In late stage (b), the mass in S8 of the liver showed contrast enhancement wash-out, and HCC was diagnosed. Hemodynamic abnormality was suspected, because wash-out was not found in the peripheral part of S7/8 of the liver. However, because the option of surgery was being considered, detailed examination was judged to be necessary.
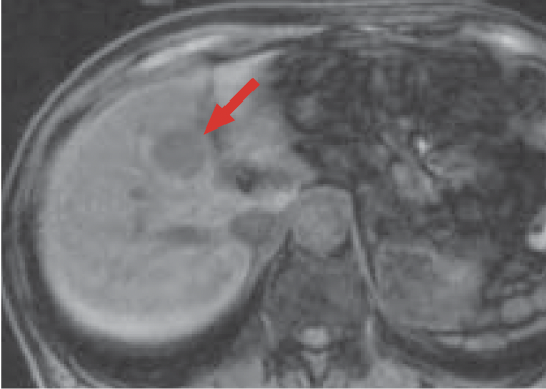
c) Pre-contrast, T1-weighted image
d) T2-weighted, fast-spin echo image
MRI
The mass (red arrow) in S8 of the liver gave a low signal in pre-contrast, T1-weighted imaging (c). In addition, in T2-weighted imaging (d) the mass gave a higher signal than the surrounding hepatic parenchyma, and its interior was somewhat heterogeneous.
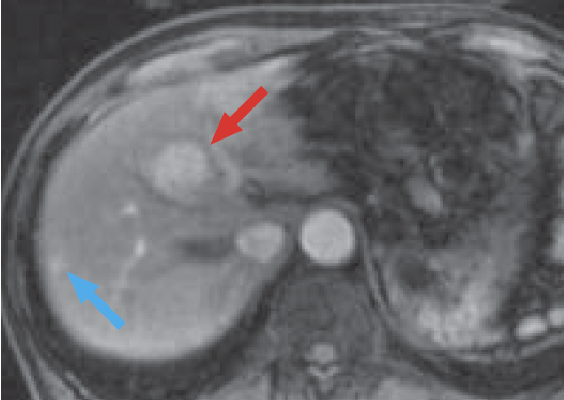
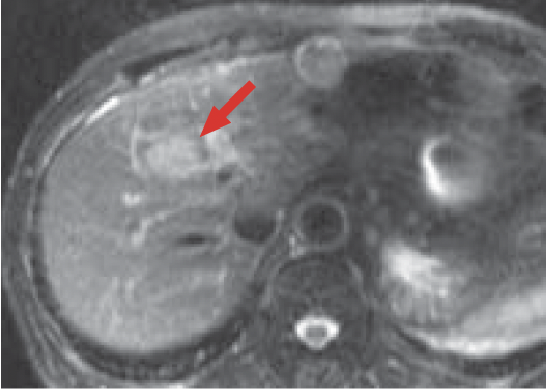
e) Arterial phase

f) Portal phase
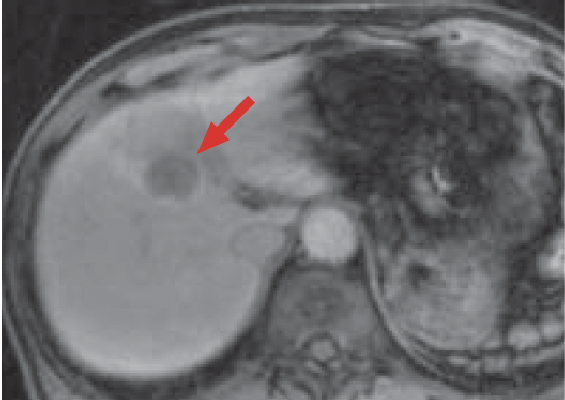
g) Late phase
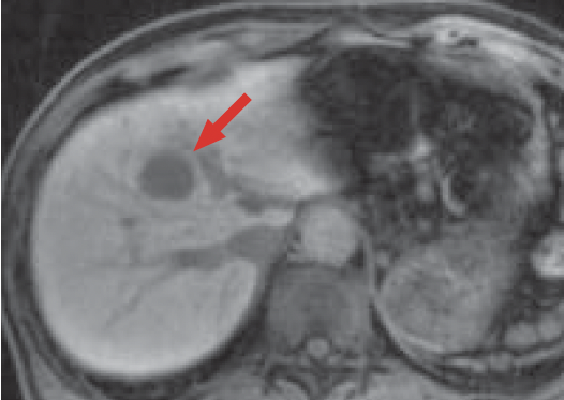
h) Hepatobiliary phase
Gadoxetate disodium(Gd-EOB-DTPA) contrast
In the Gadoxetate disodium(Gd-EOB-DTPA) contrast MRI arterial phase (e), the mass in S8 of the liver (red arrow) was stained even more intensely than the surrounding hepatic parenchyma, and it showed wash-out from the portal phase (f) to the late phase (g). In the hepatobiliary phase (h), the signal decreased markedly, and the border with the surrounding tissues became distinct. On the other hand, whereas a wedge-shaped, dark-stained region (blue arrow) was found in the periphery of S7/8 of the liver in the arterial phase (e), in the hepatobiliary phase (h) the signal was approximately the same as in the surrounding hepatic parenchyma, and hemodynamic abnormality was therefore diagnosed.
Summary
After the above, by means of surgery the tumor in S8 of the liver was diagnosed as moderately differentiated HCC.
No mass was found in the periphery of S7/8 of the liver by intraoperative ultrasonography.
It is possible to thoroughly assess hemodynamics by means of dynamic examination after Gadoxetate disodium(Gd-EOB-DTPA) administration. Typical HCC is characterized by early-phase dark staining and late-phase wash-out, and these signs were found with the present patient.
In addition, inclusion of the hepatobiliary phase resulted in visualization of the lesion, giving a distinct low signal, and this is expected to lead to increased detection effectiveness. On other hand, pseudolesions such as hemodynamic abnormalities present problems for diagnosis of HCC. Uptake of Gadoxetate disodium(Gd-EOB-DTPA) by hepatocytes makes it possible to rule out the pseudolesions that present problems for diagnosis on the basis of hemodynamics alone.
Precautions relating to administration
Administration to elderly patients
Elderly patients generally show depressed physiological function, so administration must be performed with care, and with sufficient monitoring of the patient’s condition.
- * The case introduced is just one clinical case, so the results are not the same as for all cases.
- * Please refer to the Package Insert for the effects and indications, dosage and administration method, and warnings, contraindications, and other precautions with use.


