Practical report of use for detection of hepatocellular carcinoma (HCC): Diagnosis of HCC using 3-tesla magnetic resonance imaging (3T MRI)
Tottori University Hospital
Faculty of Medicine, Tottori University
Dept. of Pathophysiological and Therapeutic Science
Division of Radiology
Drs. Suguru Kakite (current position: Deputy Director, Dept. of Radiology, Matsue Red Cross Hospital), Shinya Fujii, Yasutoshi Ota (current position: Director, Dept. of Radiology, National Cerebral and Cardiovascular Center Hospital), Yoshiko Kanasaki, Eiji Matsusue (current position: Director, Dept. of Radiology, Tottori Prefectural Central Hospital), Toshio Kaminou (current position: Deputy Director, Tsukazaki Hospital), Toshihide Ogawa
DATE : 2021
Diagnosis of HCC using 3T MRI
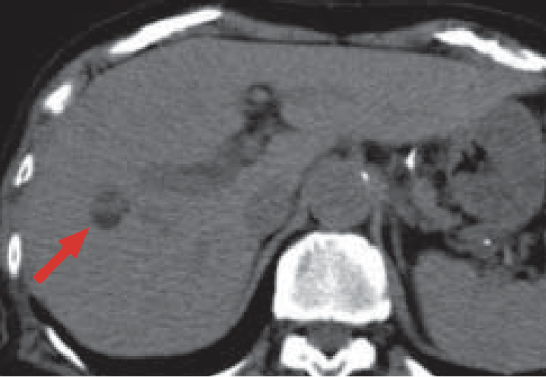
a) Pre-contrast
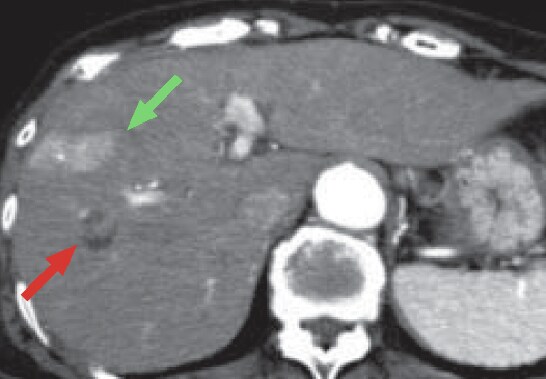
b) Arterial phase
Multidetector computed tomography
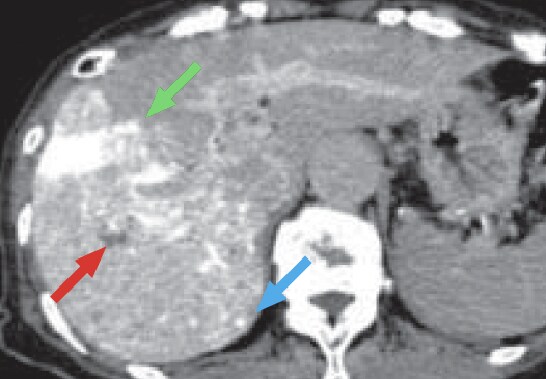
c) Computed tomography during hepatic arteriography (CTHA)
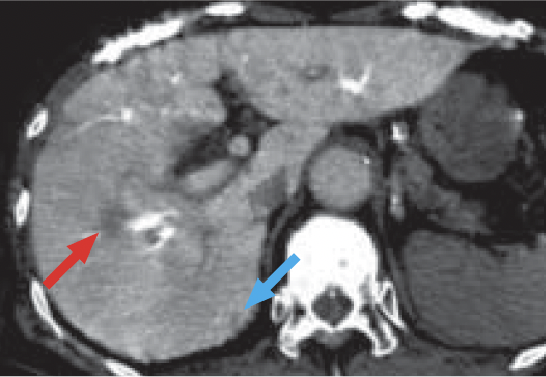
d) Computed tomography during arterial portography (CTAP)
Computed tomography (CT) during angiography
Pre-contrast CT (a) showed a low-absorption, fat-containing nodule in S8 of the liver (red arrow).
The CT arterial phase (b) showed, in S8 of the liver, a fat-containing nodule, part of which showed early-stage dark staining, suggesting HCC (red arrow).
In addition, a wedge-shaped region with early-stage dark staining was found on the lateral side of S8 (green arrow), suggesting an arterioportal shunt, but no other clear, early-stage dark staining was found.
CTHA (c) showed contrast enhancement in part of the HCC in S8 of the liver (red arrow), and a small region of dark staining in S7 of the liver (blue arrow).
In addition, similarly to CT, CTHA showed a wedge-shaped region of dark staining, suggesting an arterioportal shunt, on the lateral side of S8 of the liver (green arrow).
CTAP (d) showed a low-absorption region in S8 of the liver, which was consistent with HCC (red arrow).
A small, low-absorption region was also suspected in the margin of S7 of the liver, but it was indistinct (blue arrow).
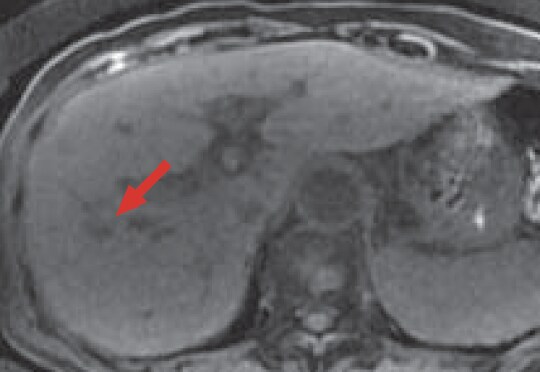
e) Pre-contrast, T1-weighted image
MRI
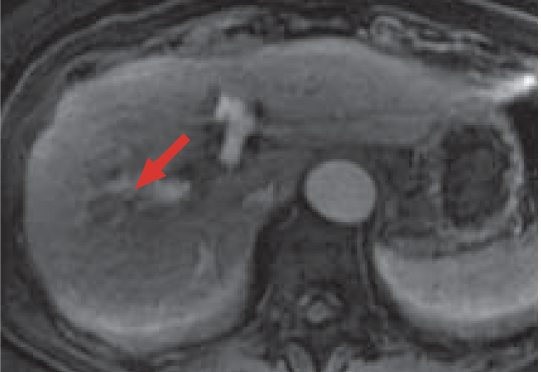
f) Arterial phase
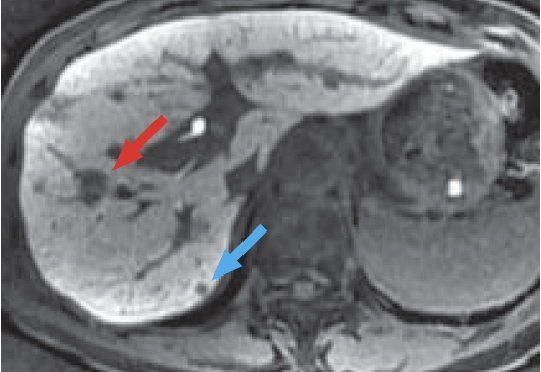
g) Hepatobiliary phase, 20 min after administration
Gadoxetate disodium(Gd-EOB-DTPA)contrast MRI
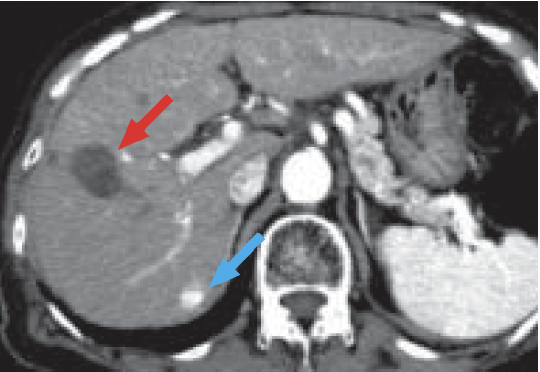
h) Arterial phase
MDCT after 3 months
In pre-contrast MRI (e), most of the HCC in S8 of the liver showed a low signal (red arrow).
In the arterial phase of Gadoxetate disodium(Gd-EOB-DTPA) contrast MRI (f), most of the HCC in S8 of the liver showed a low signal, and some of it showed slight dark staining (red arrow).
Otherwise, no clear early-stage dark staining was found.
In the hepatobiliary phase (g), the HCC in S8 of the liver showed a distinct low signal (red arrow).
In addition, a small low-signal region (blue arrow) was found in S7 of the liver, suggesting the presence of HCC.
In the CT arterial phase after 3 months (h), a scar due to radiofrequency ablation of HCC was found in S8 of the liver (red arrow).
In addition, new, early-stage dark staining was found in S7 of the liver, which was thought to indicate expansion of the nodule found by MRI (blue arrow).
Patient’s background and objectives of MRI
Female, 80s.
Abdominal ultrasonography revealed a mass in S8 of the liver, and this was diagnosed as HCC on the basis of dynamic CT.
Approximately 1 month later, MRI was performed as a thorough examination, and CT during angiography was performed after another week.
This patient constitutes a case of minute HCC that could not be identified by dynamic CT (b), and was difficult to evaluate even with CTHA (c) and CTAP (d), being visualized distinctly in the hepatobiliary phase (g) of Gadoxetate disodium(Gd-EOB-DTPA) contrast MRI.
Use of 3-tesla liver acquisition with volume acceleration (3T LAVA) in the hepatobiliary phase enabled thin-slice imaging while maintaining a high signal/noise ratio, and thus made it easier to identify a minute nodule and to distinguish it from the hepatic blood vessels. Recently, new sequences for MRI, such as LAVA, have greatly improved resolution in time, space and contrast. Combining 3T LAVA with Gadoxetate disodium (Gd-EOB-DTPA) has enabled high-resolution imaging that is clearly different from the results of conventional MRI.
Precautions relating to administration
Administration to elderly patients
Elderly patients generally show depressed physiological function, so administration must be performed with care, and with sufficient monitoring of the patient’s condition.
- * The case introduced is just one clinical case, so the results are not the same as for all cases.
- * Please refer to the Package Insert for the effects and indications, dosage and administration method, and warnings, contraindications, and other precautions with use.


