Moderately differentiated hepatocellular carcinoma (HCC):
Usefulness of EOB-MRI for HCC shown by computed tomography (CT) to have hepatic hemangiomatous contrast changes
Saitama Medical University Hospital
Drs. Kaiji Inoue and Eito Kozawa, Dept. of Radiology
Dr. Yasutaka Baba, Saitama Medical University International Medical Center
Siemens
MAGNETOM Avanto
DATE : 2021
INTRODUCTION

Patient’s background
Male; 60s; body weight: 59 kg; HCC
Investigation objectives
A hepatic tumor was detected at a different hospital, and the patient was referred to the authors’ hospital for thorough examination and treatment. Hematology tests gave a positive result for HCV, and showed α-fetoprotein to have increased to 21.6 ng/mL. As a thorough examination, abdominal ultrasonography and abdominal contrast CT were performed. The ultrasonography suggested HCC, whereas the CT suggested that hemangioma and focal nodular hyperplasia (FNH) were also possible. In order to make a definitive diagnosis, magnetic resonance imaging (MRI) was performed using EOB.
Contrast agent used
Gadoxetate disodium(Gd-EOB-DTPA) injection, 0.1 mL/kg
CASE EXPLANATION
Abdominal ultrasonography showed pulsatile blood flow in a nodule that was of moderate to high brightness, and had a halo. Abdominal contrast CT showed stronger contrast enhancement than in the hepatic parenchyma in the early and portal phases, and in the equilibrium phase no clear wash-out was found, so it was difficult to distinguish the nodule from the hepatic parenchyma. With MRI T2-weighted imaging, signal intensity was lower than in the cyst (*) in S4. With contrast dynamic MRI using EOB, stronger contrast enhancement than in the hepatic parenchyma were found from the early to late phases. In the portal phase, corona-like dark staining was found. In the hepatobiliary phase, no EOB uptake was found. HCC was judged to be highly probable, and surgery was performed.The pathological diagnosis made was moderately differentiated HCC.
IMAGING SIGNS
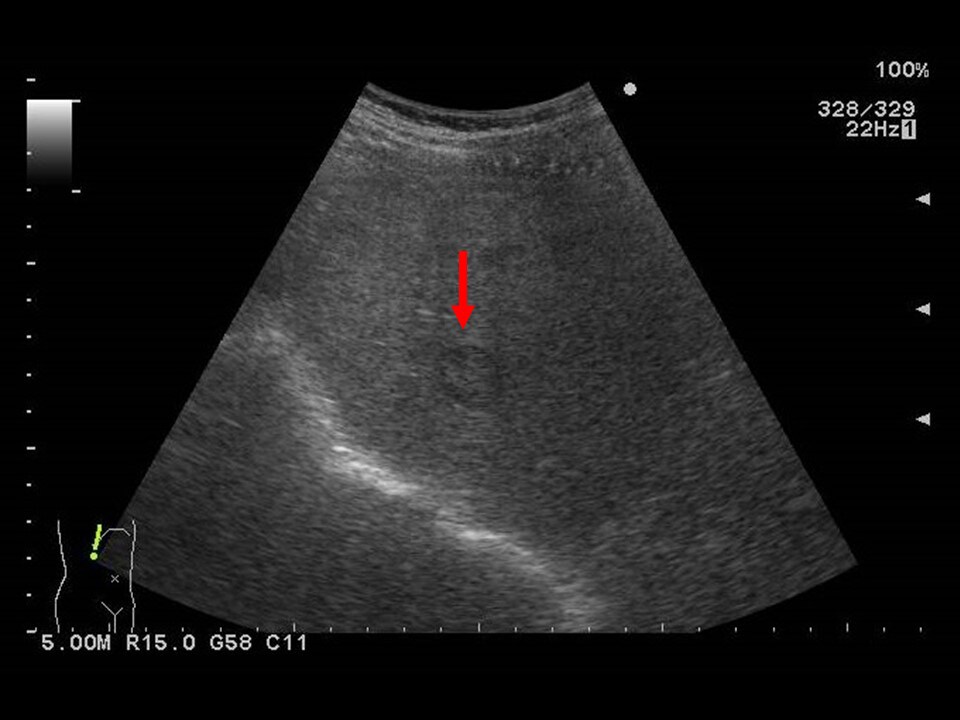
Fig. 1. Abdominal ultrasonography (1)
A nodule with surrounding halo is shown.
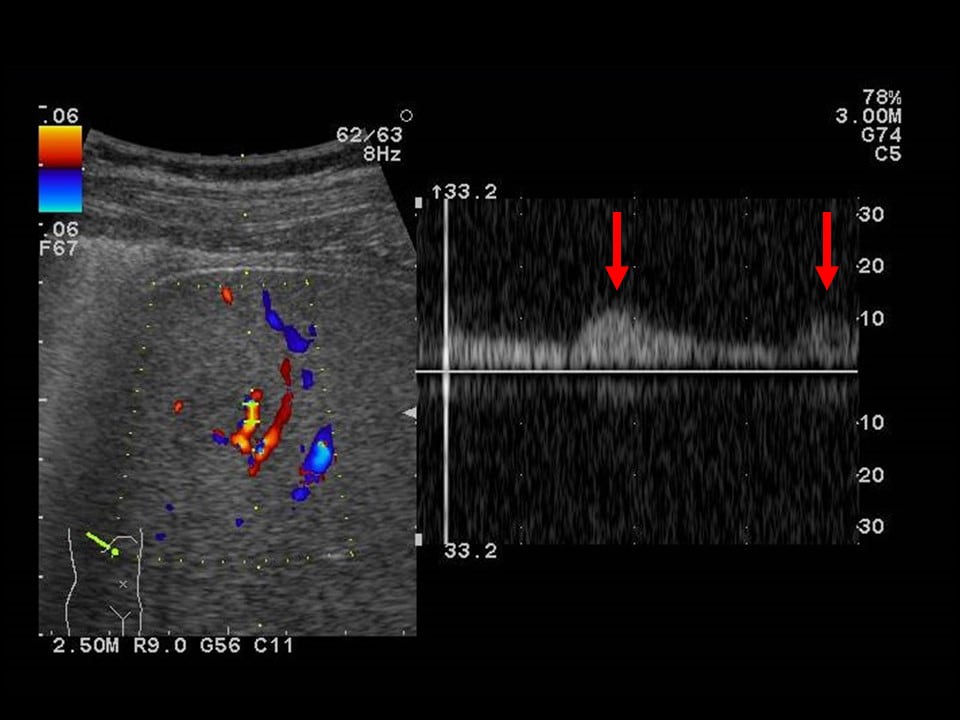
Fig. 2. Abdominal ultrasonography (2)
The signal shows highly pulsatile blood flow.
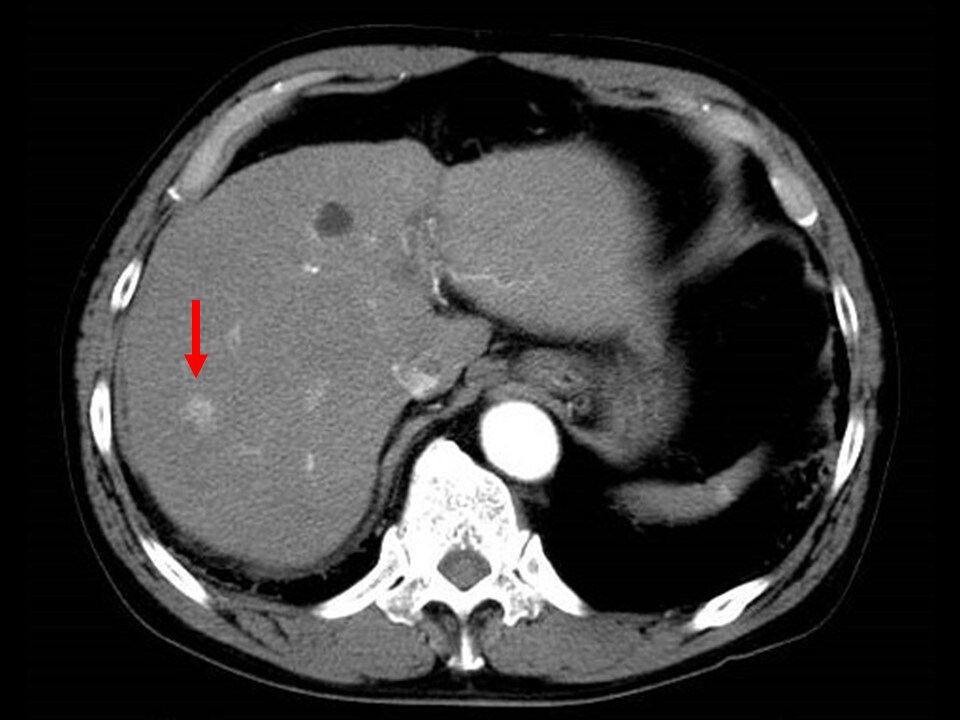
Fig. 3. Early phase of contrast CT
Intense dark staining is shown.
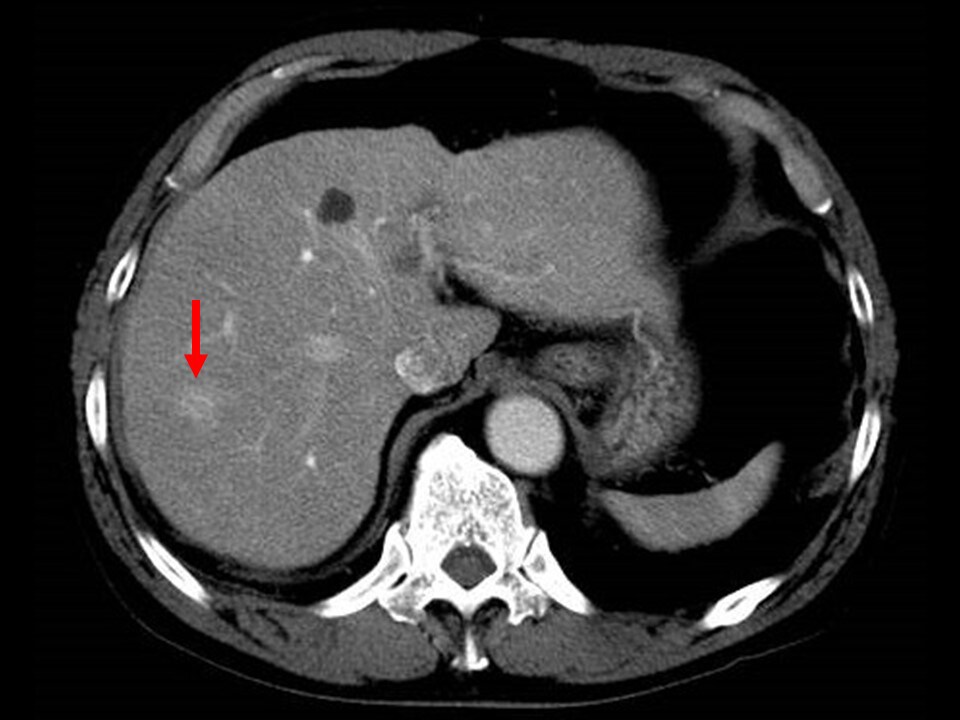
Fig. 4. Portal phase of contrast CT
More intense enhancement effects than in the hepatic parenchyma are shown.
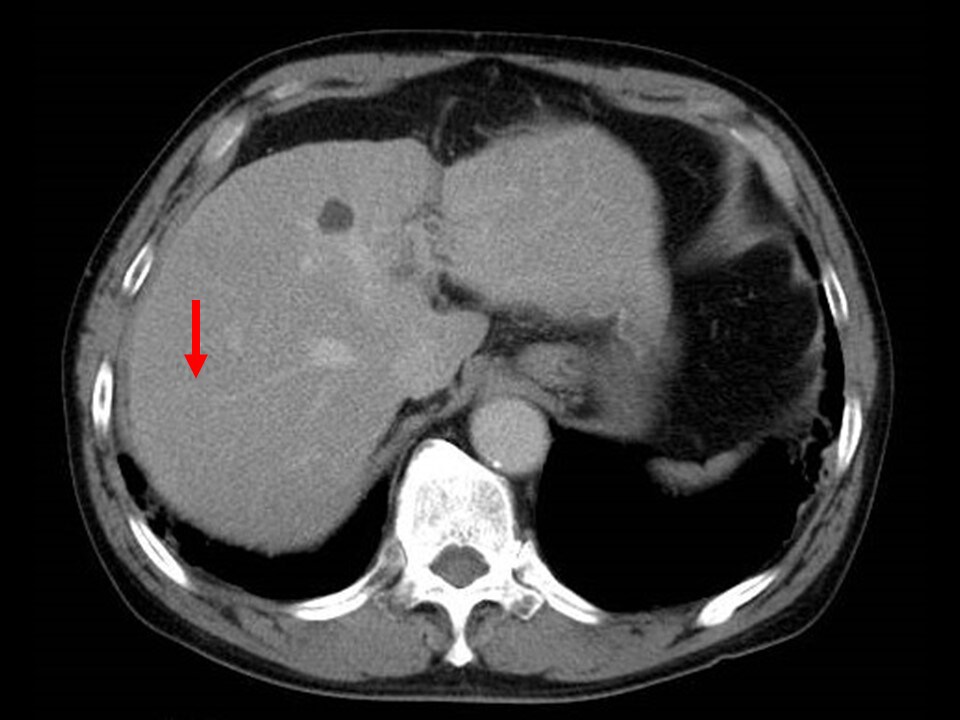
Fig. 5. Equilibrium phase of contrast CT
The tumor is difficult to distinguish from the surrounding hepatic parenchyma.
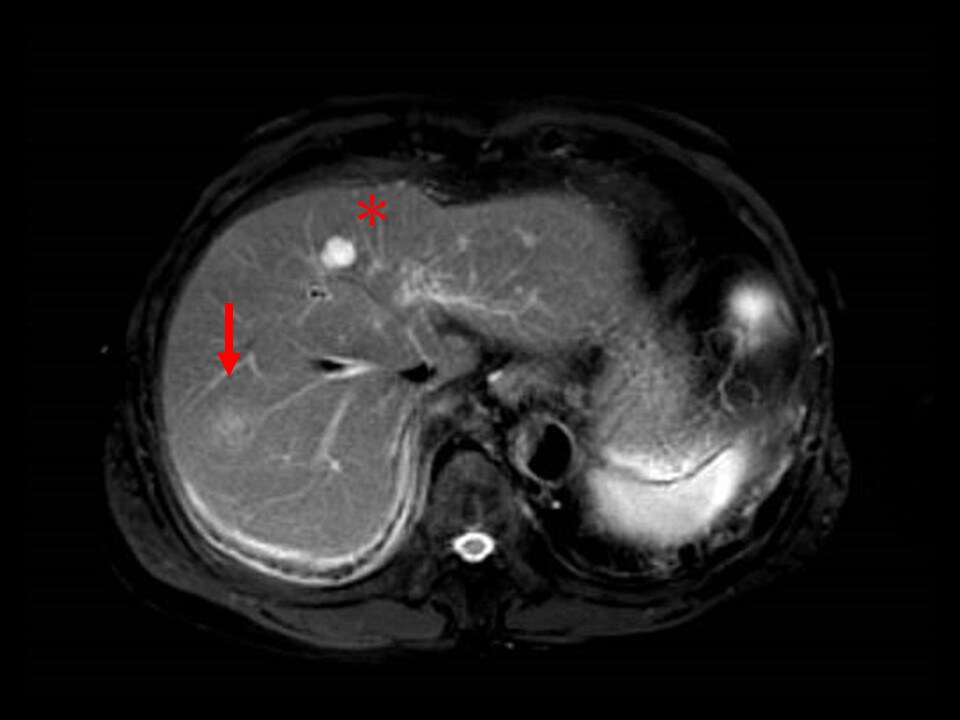
Fig. 6. MRI T2-weighted image
The nodule shows a lower signal than the cyst (*) in S4.
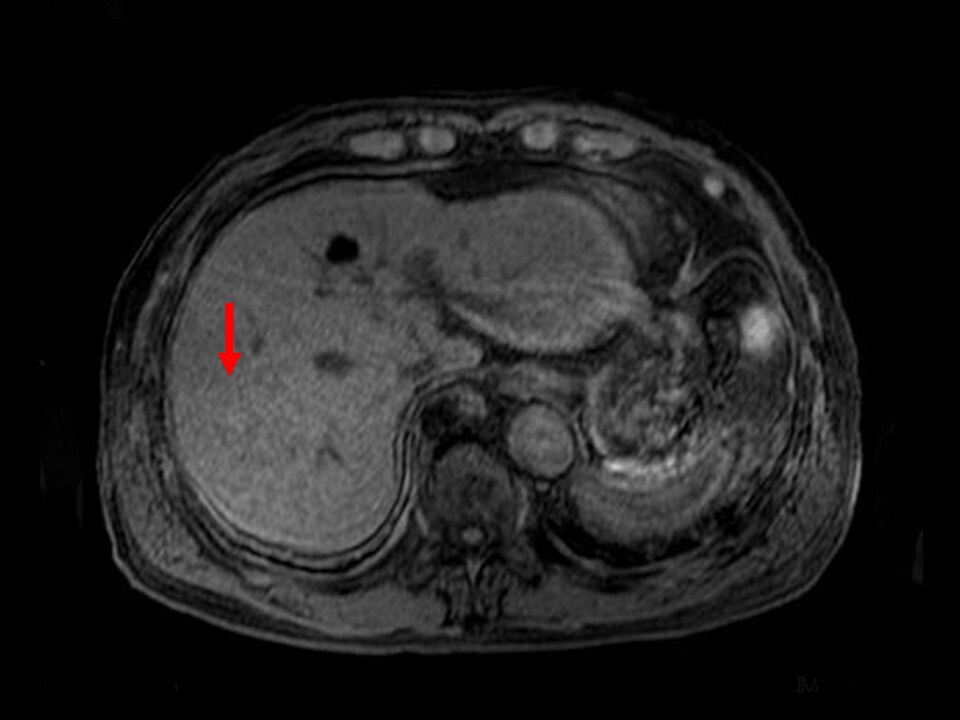
Fig. 7. T1-weighted image with simple MRI
A low-signal nodule is shown.
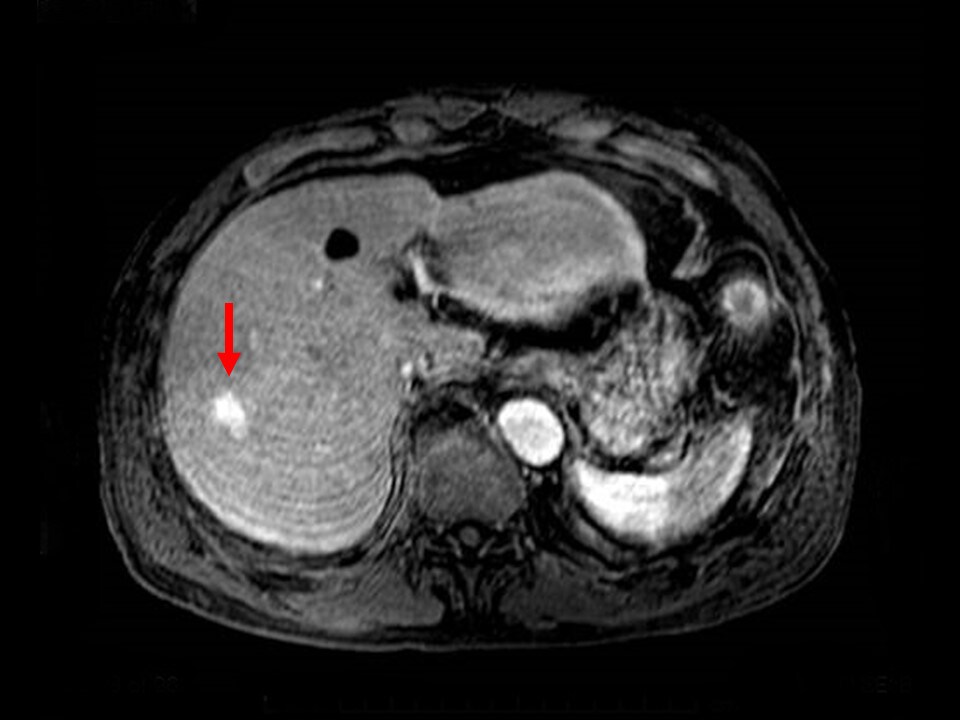
Fig. 8. T1-weighted image in MRI early phase
The contrast enhancement is more intense than in the hepatic parenchyma.
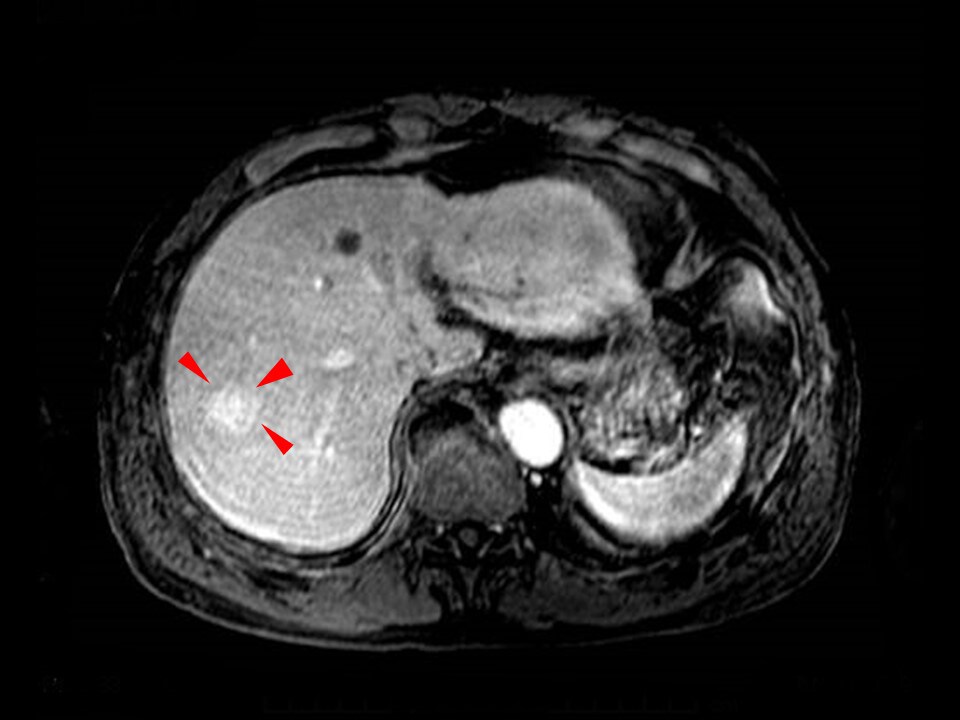
Fig. 9. T1-weighted image in MRI portal phase
The contrast signs consisted of corona-like dark staining surrounding the tumor.
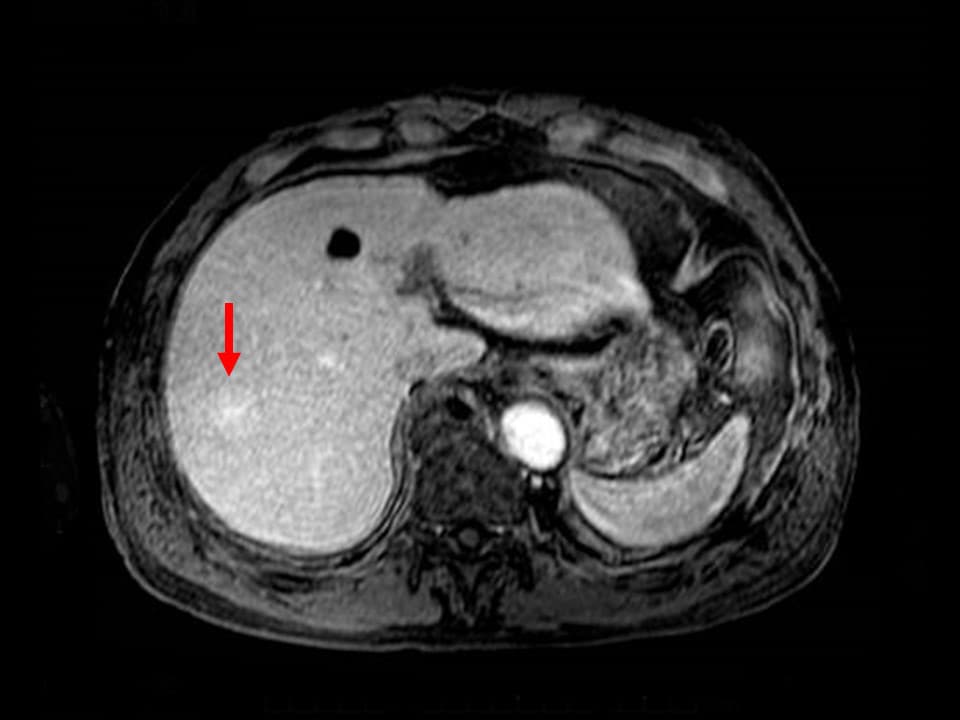
Fig. 10. T1-weighted image in MRI late phase
The enhancement effects are more intense than in the hepatic parenchyma.

Fig. 11. T1-weighted image in MRI hepatobiliary phase
There is no EOB uptake, and there is a low signal.
PHOTOGRAPHY PROTOCOL
| Imaging type | Photography sequence |
TE (ms) | TR (ms) | FA (º) | Fat sat (type) |
Holding breath |
NEX (calcula-tion number) |
|---|---|---|---|---|---|---|---|
| Dual echo | 2D FLASH | 2.14 4.83 |
195 | 75 | ー | ー | ー |
| Contrast agent | |||||||
| Dynamic | VIBE | 1.47 | 3.66 | 10 | CHESS | Yes | 1 |
| DWI | EPI | 75 | 1100 | 90 | CHESS | ー | ー |
| T2WI | FSE (BLADE) |
64 | 3291 | 150 | CHESS | ー | ー |
| Imaging type | k-space | Slice thick-ness (mm) |
FOV (mm) |
Rectangular FOV (%) |
Phase direction (step number) |
Read direction (matrix number) |
Slice gap (mm) |
Slice number |
|---|---|---|---|---|---|---|---|---|
| Dual echo | ー | 8 | 350 | ー | 205 | 253 | 2 | 23 |
| Contrast agent | ||||||||
| Dynamic | sequential | 25 | 350 | 80 | 156 | 156 | 0 | 96 |
| DWI | ー | 8 | 350 | 75 | 158 | 96 | 0 | 30 |
| T2WI | ー | 8 | 350 | ー | 256 | 256 | 2 | 23 |
Devices used and contrast conditions
| MRI device | MAGNETOM Avanto |
|---|---|
| Automatic injection device | Nemoto Sonic Shot GX |
| Work station | ー |
| Contrast conditions |
Dose (mL) | Administration rate (mL/s) | Photography timing | |
|---|---|---|---|---|
| Gadoxetate disodium(Gd-EOB-DTPA) | 6 | 1.5 | 35, 75 and 120 s after injection | |
| Physiological saline solution for flushing | 10 | 1.5 |
At the authors’ hospital, the photography timing with dynamic contrast is after 25, 70 and 120 s. The injection speed was 1.5 mL/s, and the injection speed for physiological saline solution for flushing was approximately the same.The volume of physiological saline solution was only 10 mL, but no inappropriate results such as poor contrast enhancement were found in the arterial-predominant phase.
USEFULNESS OF GADOXETATE DISODIUM(GD-EOB-DTPA) CONTRAST MRI WITH THIS PATIENT
The results of the hematology tests suggested HCC, but the values were not sufficiently high to be definitely abnormal. Abdominal ultrasonography showed a halo and a pulsatile blood-flow signal, strongly suggesting HCC. In the equilibrium phase of CT there was no wash-out, and the contrast pattern was somewhat atypical for HCC, suggesting the possibility of hemangioma or FNH. MRI T2-weighted imaging showed the nodule to have a considerably lower signal than the cyst in S4, so it was considered unlikely to be a hemangioma, as these commonly show intense high signals. In the portal phase of EOB-MRI, the nodule showed internal wash-out, and marginal enhancement effects with corona-like dark staining. In the hepatobiliary phase, the nodule was shown with a low signal, and it was considered unlikely to be FNH, and changes from the early to portal phase also ruled out it being a hemangioma. Corona-like dark staining was found in the portal phase, and a diagnosis of HCC was made. On the basis of the EOB-MRI findings, the possibility of a benign lesion, put forward on the basis of CT, was ruled out, and a diagnosis of HCC was finally made.
- *The case introduced is just one clinical case, so the results are not the same as for all cases.
- *Please refer to the Package Insert for the effects and indications, dosage and administration method, and warnings, contraindications, and other precautions with use.


